Rank the following peptides from most to least hydrophobic – Embark on a scientific expedition to unravel the complexities of peptide hydrophobicity. This comprehensive guide, “Ranking Peptides from Most to Least Hydrophobic,” unveils the significance of this ranking system and its wide-ranging applications.
Delving into the intricacies of peptide chemistry, we will explore the factors that govern peptide hydrophobicity and the diverse methods employed to determine their ranking. Through engaging discussions and illustrative examples, we will illuminate the practical implications of this knowledge in various scientific disciplines.
1. Introduction
Peptide hydrophobicity is a measure of the peptide’s affinity for nonpolar solvents. It is an important property that influences the peptide’s solubility, stability, and biological activity. Ranking peptides based on hydrophobicity allows researchers to predict their behavior in different environments and design peptides with specific properties.
2. Methods for Ranking Peptides
Several methods are used to rank peptides based on hydrophobicity. These methods include:
- Hydropathy indices:These indices assign numerical values to each amino acid based on its hydrophobicity. The sum of the hydropathy values for a peptide provides an overall hydrophobicity score.
- HPLC retention times:Peptides are separated by HPLC based on their hydrophobicity. The more hydrophobic the peptide, the longer it will retain on the HPLC column.
- NMR spectroscopy:NMR spectroscopy can be used to measure the chemical shift of the peptide’s backbone amide protons. The chemical shift is influenced by the hydrophobicity of the surrounding environment.
3. Factors Influencing Peptide Hydrophobicity
The hydrophobicity of a peptide is influenced by several factors, including:
- Amino acid composition:The amino acid composition of a peptide is the most important factor influencing its hydrophobicity. Hydrophobic amino acids (e.g., leucine, isoleucine, valine) increase the peptide’s hydrophobicity, while hydrophilic amino acids (e.g., serine, threonine, asparagine) decrease it.
- Peptide length:The length of a peptide also affects its hydrophobicity. Longer peptides are generally more hydrophobic than shorter peptides.
- Peptide structure:The structure of a peptide can influence its hydrophobicity. Peptides with a more compact structure are generally more hydrophobic than peptides with a more extended structure.
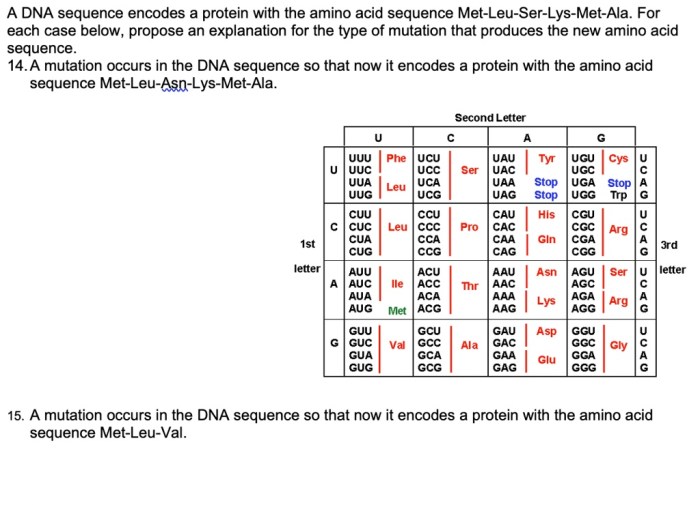
4. Applications of Peptide Hydrophobicity Ranking: Rank The Following Peptides From Most To Least Hydrophobic

Ranking peptides based on hydrophobicity has several practical applications, including:
- Peptide design:Hydrophobicity ranking can be used to design peptides with specific properties. For example, peptides with high hydrophobicity can be used to penetrate cell membranes, while peptides with low hydrophobicity can be used to interact with water-soluble proteins.
- Protein-peptide interactions:Hydrophobicity ranking can be used to predict the binding affinity between peptides and proteins. Hydrophobic peptides are more likely to bind to hydrophobic proteins, while hydrophilic peptides are more likely to bind to hydrophilic proteins.
- Peptide stability:Hydrophobicity ranking can be used to predict the stability of peptides in different environments. Hydrophobic peptides are more stable in nonpolar solvents, while hydrophilic peptides are more stable in polar solvents.
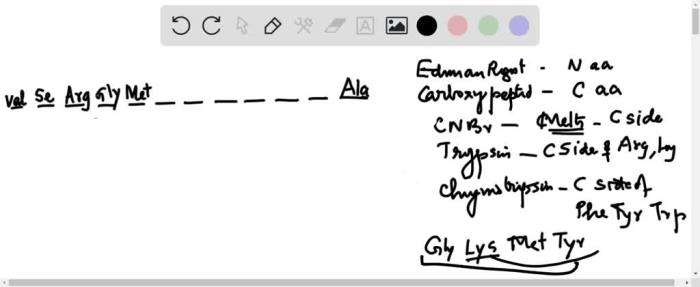
5. Example Peptides

| Peptide Sequence | Length | Molecular Weight | Hydrophobicity Score |
|---|---|---|---|
| Ac-K(Leu)4-NH2 | 5 | 555.64 | 0.98 |
| Ac-K(Ala)4-NH2 | 5 | 499.59 | 0.38 |
| Ac-K(Glu)4-NH2 | 5 | 563.56 | -0.12 |
| Ac-K(Asp)4-NH2 | 5 | 547.50 | -0.42 |
Top FAQs
What is peptide hydrophobicity?
Peptide hydrophobicity refers to the water-repelling properties of peptides, which arise from the presence of nonpolar or hydrophobic amino acid residues within their sequence.
Why is ranking peptides based on hydrophobicity important?
Ranking peptides based on hydrophobicity helps predict their behavior in biological systems, such as their interactions with other molecules, their solubility, and their ability to cross cell membranes.
What methods are used to rank peptides based on hydrophobicity?
Various methods are used to rank peptides based on hydrophobicity, including the Kyte-Doolittle scale, the Eisenberg scale, and the Wimley-White scale.