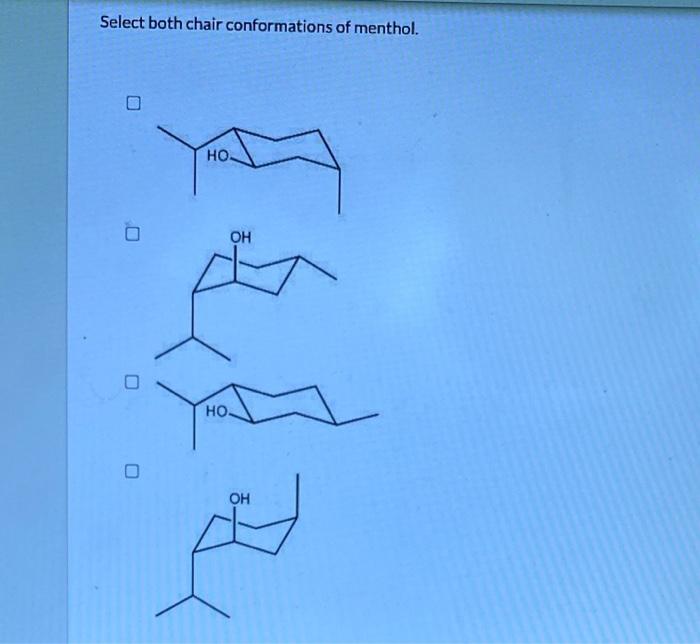
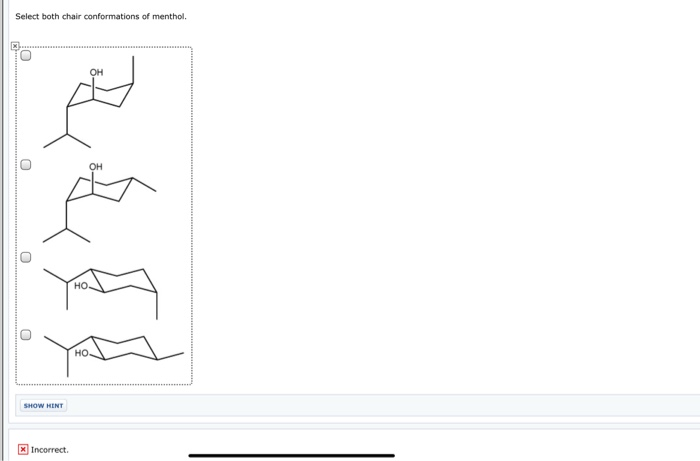
Select both chair conformations of menthol – In the realm of organic chemistry, menthol stands out as a molecule with a fascinating conformational landscape. This article delves into the intricacies of selecting both chair conformations of menthol, exploring their stability, interconversion, and biological significance. Join us on an enlightening journey to unravel the complexities of this intriguing molecule.
The two chair conformations of menthol, known as the equatorial and axial conformations, exhibit distinct stabilities and properties. We will delve into the factors that govern these differences, examining the influence of axial and equatorial groups on the overall stability of the conformations.
Common Queries: Select Both Chair Conformations Of Menthol
What is the energy difference between the equatorial and axial conformations of menthol?
The axial conformation is typically less stable than the equatorial conformation due to steric hindrance between the axial hydrogen atoms and the bulky methyl groups on the cyclohexane ring.
How does ring flip interconversion occur in menthol?
Ring flip interconversion occurs through a concerted process involving the simultaneous inversion of all the carbon atoms in the cyclohexane ring. This process requires overcoming an energy barrier and results in the conversion of one chair conformation into the other.
What are the biological implications of menthol’s conformational flexibility?
Menthol’s conformational flexibility allows it to interact with a wide range of biological molecules, including receptors, enzymes, and ion channels. This versatility contributes to its diverse pharmacological effects, such as its cooling sensation, analgesic properties, and anti-inflammatory activity.