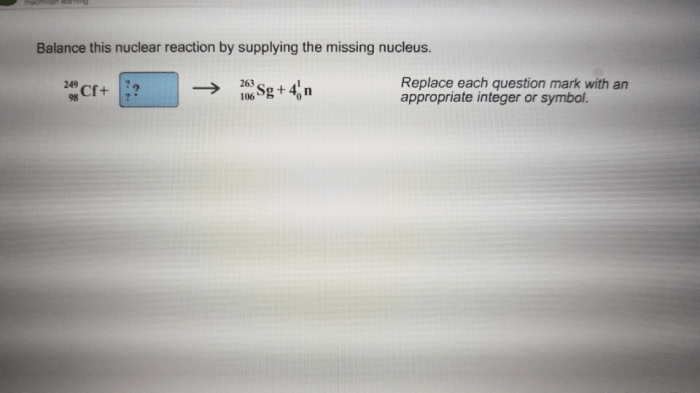
Balance this nuclear reaction by supplying the missing nucleus, a captivating journey into the realm of nuclear physics, where we explore the fundamental principles of balancing nuclear reactions and unveil the significance of identifying the missing nucleus. This discourse delves into the practical applications of nuclear reaction balancing, showcasing its profound impact on fields ranging from nuclear power generation to medical imaging.
Nuclear Reaction Balancing
Nuclear reactions involve the transformation of one or more atomic nuclei into different nuclei. Balancing nuclear reactions is crucial to ensure that the principles of conservation of mass and charge are upheld.
Atomic numbers, representing the number of protons, and mass numbers, representing the sum of protons and neutrons, play a vital role in balancing nuclear reactions. The total atomic number and mass number on the reactant side must equal those on the product side.
Missing Nucleus Identification
Identifying the missing nucleus in a nuclear reaction requires an understanding of the principles of conservation of mass and charge. The mass number of the reactants must equal the mass number of the products, and the atomic number of the reactants must equal the atomic number of the products.
By applying these principles, it is possible to determine the missing nucleus. For instance, if we have the reaction 14N + 4He → ?, the missing nucleus must have an atomic number of 8 (14 + 4 = 8) and a mass number of 17 (14 + 4 = 17). This corresponds to the nucleus 17O.
Balancing Nuclear Reactions
To balance a nuclear reaction, we need to identify the missing nucleus and ensure that the atomic numbers and mass numbers are balanced on both sides of the equation. The following steps can be used:
- Write the unbalanced nuclear reaction.
- Identify the missing nucleus using the principles of conservation of mass and charge.
- Balance the atomic numbers by adjusting the subscripts of the elements.
- Balance the mass numbers by adjusting the superscripts of the elements.
- Check if the reaction is balanced by verifying that the total atomic numbers and mass numbers are the same on both sides.
Applications of Nuclear Reaction Balancing, Balance this nuclear reaction by supplying the missing nucleus
Nuclear reaction balancing has numerous practical applications, including:
- Nuclear power plants:Balancing nuclear reactions is essential for determining the optimal fuel composition and predicting the energy output of nuclear reactors.
- Medical imaging:Balanced nuclear reactions are used in techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) to diagnose and treat medical conditions.
- Scientific research:Balancing nuclear reactions enables scientists to study the properties of atomic nuclei, investigate nuclear processes, and develop new technologies.
Popular Questions: Balance This Nuclear Reaction By Supplying The Missing Nucleus
What is the significance of balancing nuclear reactions?
Balancing nuclear reactions is essential to ensure the conservation of mass and charge, fundamental principles in nuclear physics.
How do we identify the missing nucleus in a nuclear reaction?
The missing nucleus can be identified by applying the principles of conservation of mass and charge, comparing the atomic and mass numbers of the reactants and products.
What are the practical applications of balancing nuclear reactions?
Balancing nuclear reactions has applications in nuclear power plants, medical imaging, and scientific research, contributing to advancements in energy production, medical diagnostics, and our understanding of the universe.